Chapter 3. Theory
There are several methods available for measuring blood cell velocity within single capillaries: flying spot, frame to frame, and photometric correlation. They are all image based techniques and can only measure flows in vessels lying parallel to the surface. Since the measurement is derived from the image, good, high contrast images are necessary. This is not always possible to obtain in many subjects and using standard video equipment restricts the maximum range of measurement to the order of 2 mm/s.
Measuring velocities using the laser Doppler technique is well established and there are many applications in the study of fluid flow. The technique was first demonstrated in 1964 (YEH et al., 1964).
The first in vivo application of laser Doppler was the measurement of the velocity of blood cells in an 80 micron diameter retinal artery of an albino rabbit (RIVA et al., 1972). In 1974 Mishina et al. described a dual beam Laser Doppler Microscope (Mishina et al.,1974). This was used to demonstrate the measurement of velocity in a 70 micron diameter venule in the web of a frog's foot by transmission through the tissue (Mishina et al., 1976). Another laser Doppler microscope anemometer (Einav et al., 1975) was used to measure velocity profiles in arterioles 65-98 microns in diameter.
All the above techniques measure velocities parallel to the tissue surface, which like the imaging techniques restricts measurements to such sites as the nailfold or forearm.
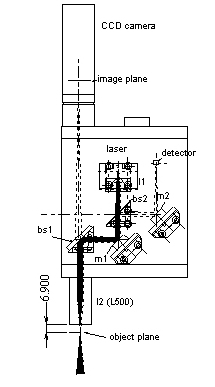
The CAM1 will measure red blood cell velocities in small vessels, particularly the capillaries of skin, but also the surface capillaries of any organ. Skin tissue is relatively transparent and the apex of capillaries can be viewed using a microscope with high illumination. For maximum contrast between red blood cells and the surrounding tissue, green light of 525 nm is used.
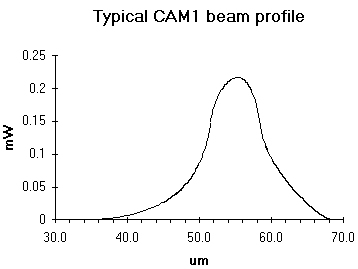
A near infrared (780nm) laser produces a 5x10 μm elliptical spot, with a power of about 1 mW.
When the CAM1 is positioned and focused so that the laser beam is on a vertical arterial or venous limb of a capillary loop, a small fraction of the laser light will be backscattered by the column of blood cells and collected by lens l2. Lens l2 will also collect laser light reflected from the surrounding tissue.
Laser light backscattered by a moving blood cell will shift the frequency of the light. The amount of the shift will be proportional to blood cell velocity:
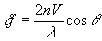
where n is the refractive index of the medium (1.33) and λ is the laser wavelength (780 nm).
The maximum frequency shift obtainable at any capillary blood cell velocity is when θ is 0 or 180ℴ. Note that even at an angle of 18ℴ the shift will be down by only 5%. Of course this cosine dependency also exists for imaging techniques also, such as frame to frame and video correlation techniques.
In practice, an operator will adjust the laser beam position for the strongest signal. This will occur when there are a maximum number of blood cells present in the sample volume. In small capillaries, a perpendicular section will provide the maximum number of blood cells in the focal point, since the laser beam has a greater depth of focus than its diameter. Therefore the use of a perpendicular section is generally assured.
At the photodetector, Doppler shifted and unshifted laser light will mix to produce an electrical output with an ac component at a frequency of the difference between the shifted and unshifted light, i.e. the at the Doppler shift.
The electrical signal from the photodetector is amplified and filtered within the CAM1. The output from the CAM1 is converted by the CAM1/PC interface card at up to 100,000 samples per second.
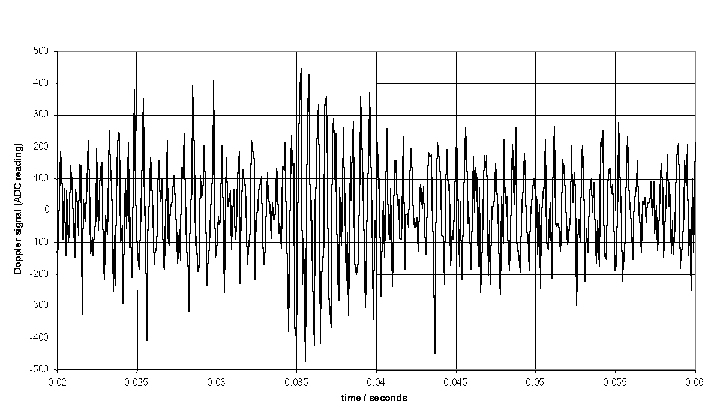
A sample of Doppler shift is shown. The high frequency Doppler shift can be clearly seen, along with low frequency amplitude variations due to the transit of the blood cell through the sample volume. In situations with large gaps between blood cells the Doppler shift component will be weak and the lower frequency components will dominate. This can cause problems at low flows.
When there is a continuous stream of blood cells these produce bursts with random amplitude and phase. This leads to a broadening of the power spectrum, and a lot of power in the 'pedestal' component of the Doppler spectrum.
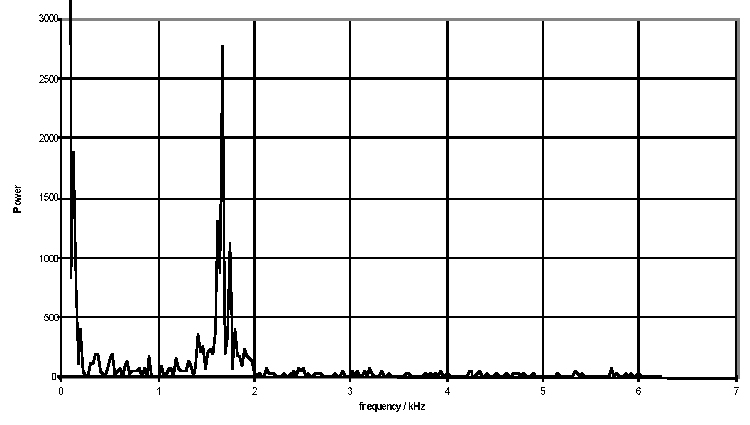
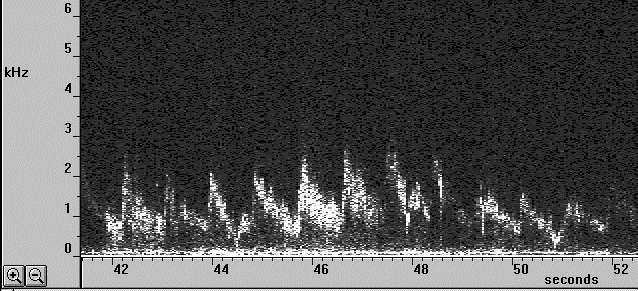
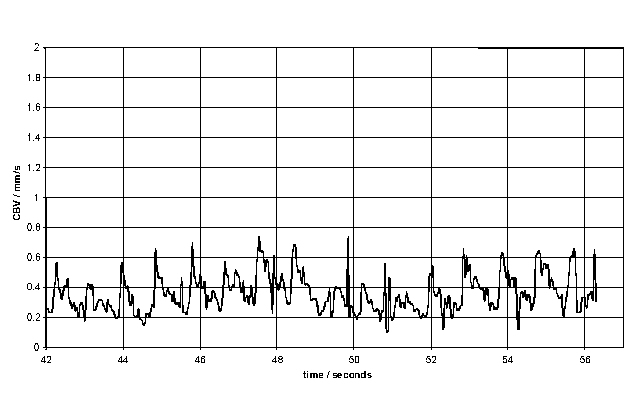
The resulting Doppler shift power spectrum (DSPS) from this signal is shown above, with a well defined narrow peak. The power scale is uncalibrated. The figure below shows how the DSPS is displayed by the CAM1 software. The raw data shown above is taken from one sample point at about the 44 seconds point in the figures below. The highly pulsatile nature of the CBV in capillaries can be seen. The calculated CBV trace is shown below right.
First results using the CAM1 have been published by MORRIS et al., 1996; STÜCKER et al., 1996; and ALTMEYER et al., 1997.
References
Altmeyer P., Hoffmann K., Stücker: 'Kutane Mikrozirkulation', Springer, ISBN 3-540-62564-X, pp 188-199.
Bollinger A., Fagrell B. (Eds.)(1990): 'Clinical Capillaroscopy'. Hogrefe & Huber Publishers.
Bonner, R. F., Clem, T. R., Bowen, P. D. and Bowman, R. L. (1981): 'Laser-Doppler continuous real-time monitor of pulsatile and mean blood flow in tissue microcirculation', in Born G. V. R., Melling A., Whitelaw J. H. (1978): 'Laser Doppler microscope for blood velocity measurements'. Biorheology, 15. pp 163-172.
Chen, S., Chu, B. and Nossal, R. (Eds.): 'NATO advanced study institutes series, Series B physics, (Plenum Press New York, USA) pp 685-701.
Cochrane T., Earnshaw J C., (1978): 'Practical laser Doppler microscopes'. J. Phys. E: Sci. Instrum., Vol 11. pp196-198.
Drain L E. (1980): 'The Laser Doppler Technique'. John Wiley & Sons.
Einav S., Berman H. J., Fuhro R. L., DiGiovanni P. R., Fridman J. D., Fine S. (1975): 'Measurement of blood flow in vivo by laser Doppler anemometry through a microscope'. Biorheology, 12. pp 203-205.
Einav S., Berman H. J., Fuhro R. L., DiGiovanni P. R., Fine S., Fridman J. D. (1975): 'Measurement of velocity profiles of red blood cells in the microcirculation by laser Doppler anemometry'. Biorheology, 12. pp 207-210.
Einav S., Berman H. J. (1988): 'Fringe mode transmittance laser Doppler microscope anemometer: its adaptation for measurement in the microcirculation'. J. Biomed. Eng. 10. Pp 393-399.
Einav S., Berman H. J., Dean H. C. (1989): 'Fringe mode reflectance laser Doppler microscope system'. J. Biomed. Eng. 11. pp 57-62.
Eiju T., Matsuda K., Ohtsubo J., Honma K., Shimizu K., (1981): 'A frequency shifting of LDV for blood velocity measurements by a moving wedged glass'. Applied Optics Vol 20. No.22. pp 3833-3837.
Eiju T., Nagai M., Matsuda K., Ohtsubo J., Homma K., Shimizu K., (1993): 'Microscopic laser Doppler velocimeter for blood velocity measurement'. Optical Engineering 32(1), pp15-20.
Holloway, G. A. and Watkins, D. W. (1977): 'Laser Doppler measurement of cutaneous blood flow', J. Invest. Dermatol., 69, 306-309.
Koyama T., Horimoto M., Mishina H., Asakura T. (1982): 'Measurements of blood flow velocity by means of a laser Doppler microscope'. Optik, 61(4), pp 411-426.
Meyer M.F., Schatz H. (1998). 'INFLUENCE OF METABOLIC CONTROL AND DURATION OF DISEASE ON MICROVASCULAR DYSFUNCTION IN DIABETES ASSESSED BY LASER DOPPLER ANEMOMETRY'. Exp Clin Endocrinol Diabetes106 pp 395-403.
Mishina H., Asakura T., Nagi S., (1974): 'A Laser Doppler Microscope'. Opt. Commun. 11. pp99-102.
Mishina H., Ushizaka T., Asakura T. (1976): 'A laser Doppler microscope'. Optics and Laser Technology, June. pp 121-127.
Morris S J, Kunzek S, Shore A C. (1996) The effect of acetylcholine on finger capillary pressure and capillary flow in healthy volunteers Journal of Physiology (1996), 494.1, pp 307-313.
Nilsson, G. E., Tenland, T. and ÖBERG, P. Ċ (1980): 'A new instrument for continuous measurement of tissue blood flow by light beating spectroscopy', IEEE Trans., BME-27, 12-19.
Nilsson, G. E. (1984): 'Signal processor for laser Doppler tissue flowmeters', Med. & Biol. Eng. & Comput., 22, 343-348.
Petrig B L., Riva C E., Grunwald J E., (1984): 'Computer Analysis of Laser Doppler Measurements in retinal Blood Vessels'. Invest. Ophthalmol. Vis. sci. 25(Suppl.), pp7.
Petrig B. L., Riva C. E. (1988): 'Retinal laser Doppler velocimetry: towards its computer-assisted clinical use'. Applied Optics. Vol 27 No 6. pp 1126-1134.
Riva C., Ross B., Benedek G. (1972): 'Laser Doppler measurements of blood flow in capillary tubes and retinal arteries' Investigative Ophthalmology November 1972. pp 936-944.
Stern, M. D. (1975): 'In vivo evaluation of microcirculation by coherent light scattering', Nature, 254, 56-58.
Stücker M, Baier V, Reuther T, Hoffmann K, Kellam K, Altmeyer P. (1996): Capillary Blood Cell Velocity in Human Skin Capillaries Located Perpendicularly to the Skin Surface: Measured by a new Laser Doppler Anemometer Microvascular Research 52, pp 188-192.
Ushizaka T., Asakura T., (1983): 'Measurements of flow velocity in a microscopic region using a transmission grating'. Applied Optics Vol 22 No 12. pp1870-1874.
Yeh Y., Cummins H. Z., (1964): Applied Physics Letters, 4, pp176-8.