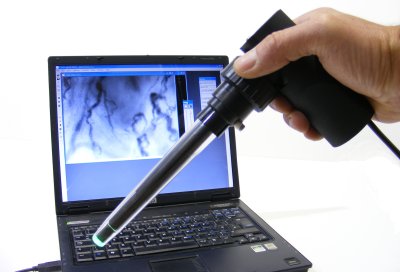
Instrumentation and software systems for analysis of the microcirculation
|Welcome| - |CapiScope CAM1 Capillary Anemometer| - |CapiScope HVCS Video Capillaroscopy| - |Sample Images and Videos| - |User Guides| - |References| - |Customers| - |Downloads| - |Contacts|
CapiScope HVCS Handheld Video Capillaroscopy System
Handheld sublingual
- Includes high quality monochrome USB camera.
- Simple single USB connection for video data and power.
- Includes narrow band safe low voltage cold light epi-illumination for high contrast images.
- Single Use plastic caps.
- Range of interchangeable lenses available for magnifications from 2x to 10x.
- Strobed illumination reduces blood cell blurring.
- Small and lightweight.
- CapiScope capillaroscopy analysis software included.
- Improved image quality and function replacement for Cytometrics OPS Cytoscan.
- Improved image quality and function replacement for Microvision Microscan.
Click on images to play video clip.
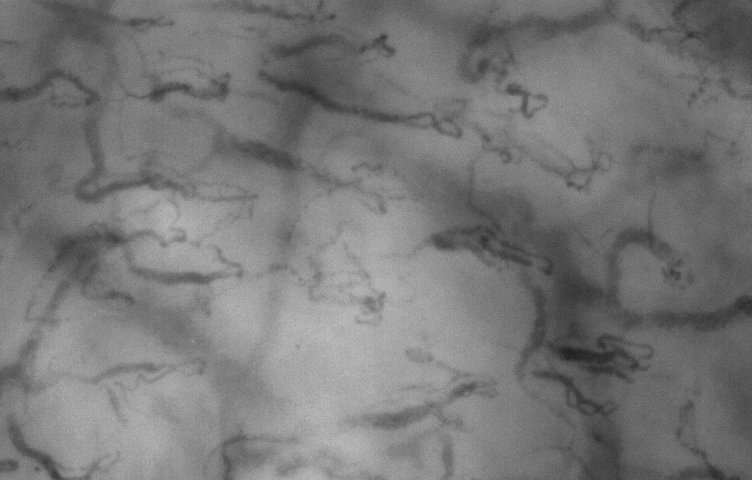 sublingual. low mag
lens.
sublingual. low mag
lens. |
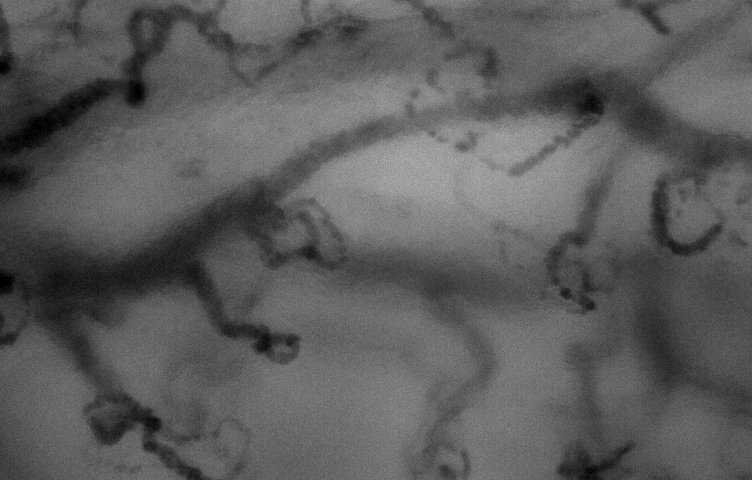 sublingual. high mag
lens.
sublingual. high mag
lens. |
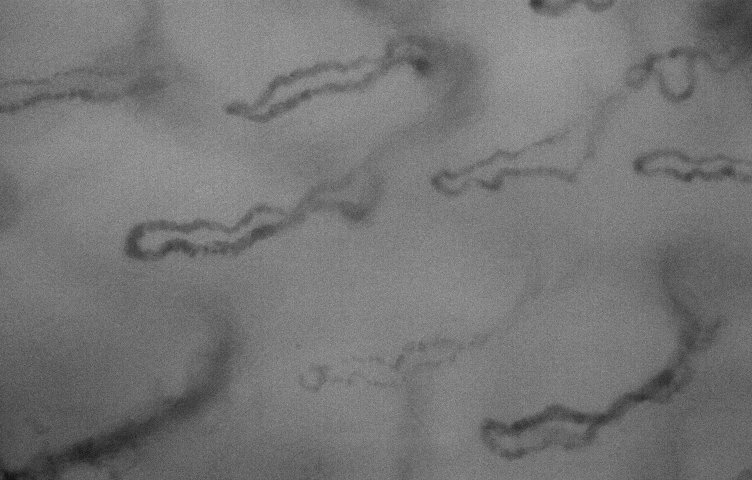 lip. high mag lens.
lip. high mag lens. |
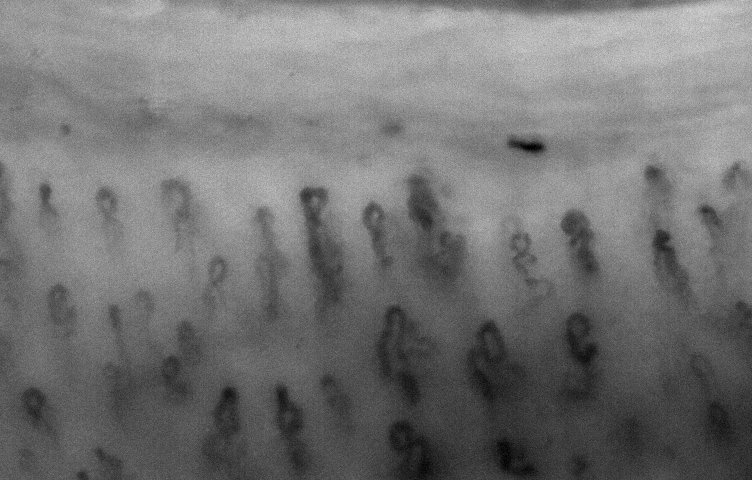 nailfold. low mag
lens.
nailfold. low mag
lens. |
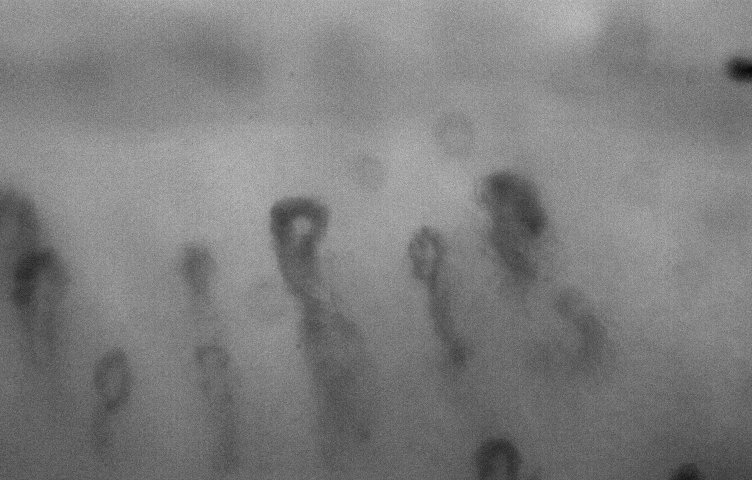 nailfold. high mag
lens.
nailfold. high mag
lens. |
Note that CapiScope records video in an uncompressed raw format. Compression used for these examples will have introduced artefacts.
Also note that the CapiScope movement correction is also not shown.
Nailfold
- Visualize, capture and record capillaries digitally.
- Simple single USB2 connection for video and power.
- Strobed illumination for sharp blur free images
- Includes CapiScope analysis software
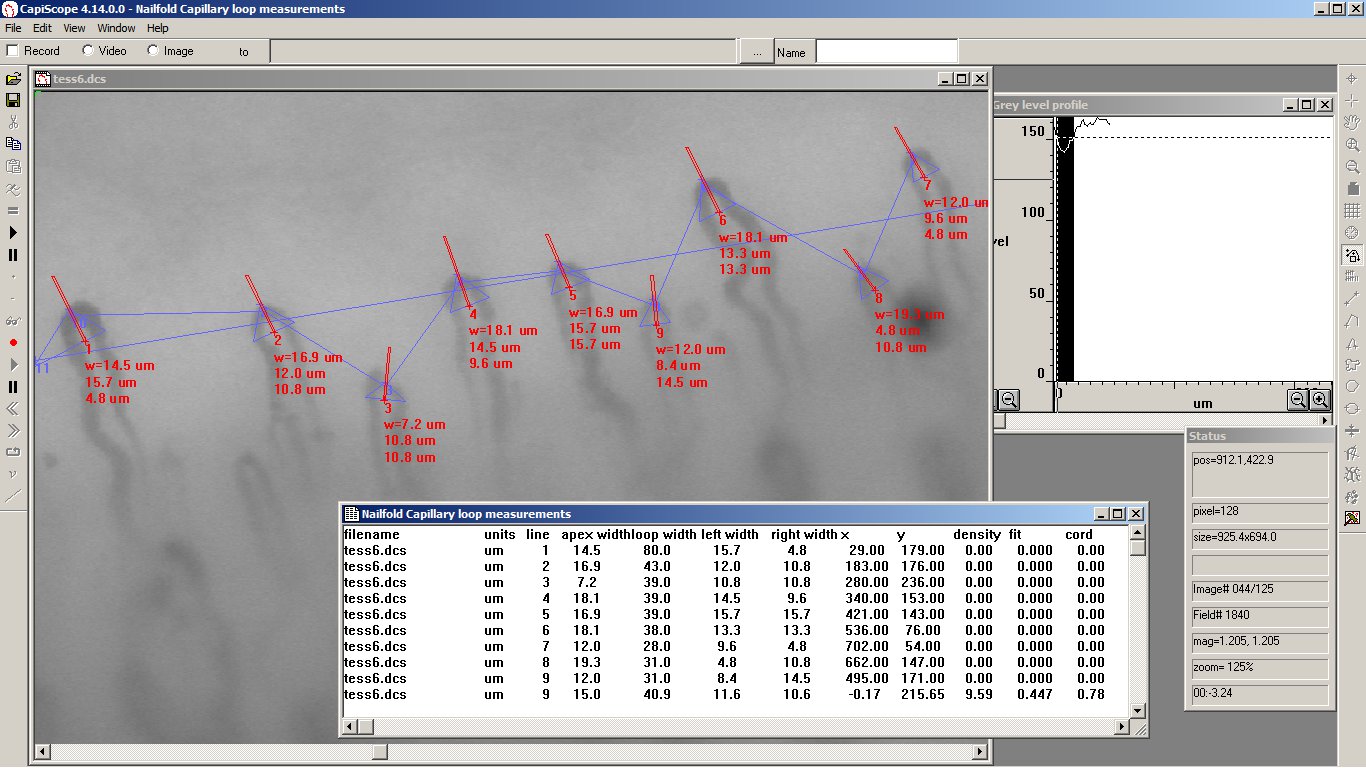
- Automatic measurement of vessel diameters.
- Manual and automatic capillary density.
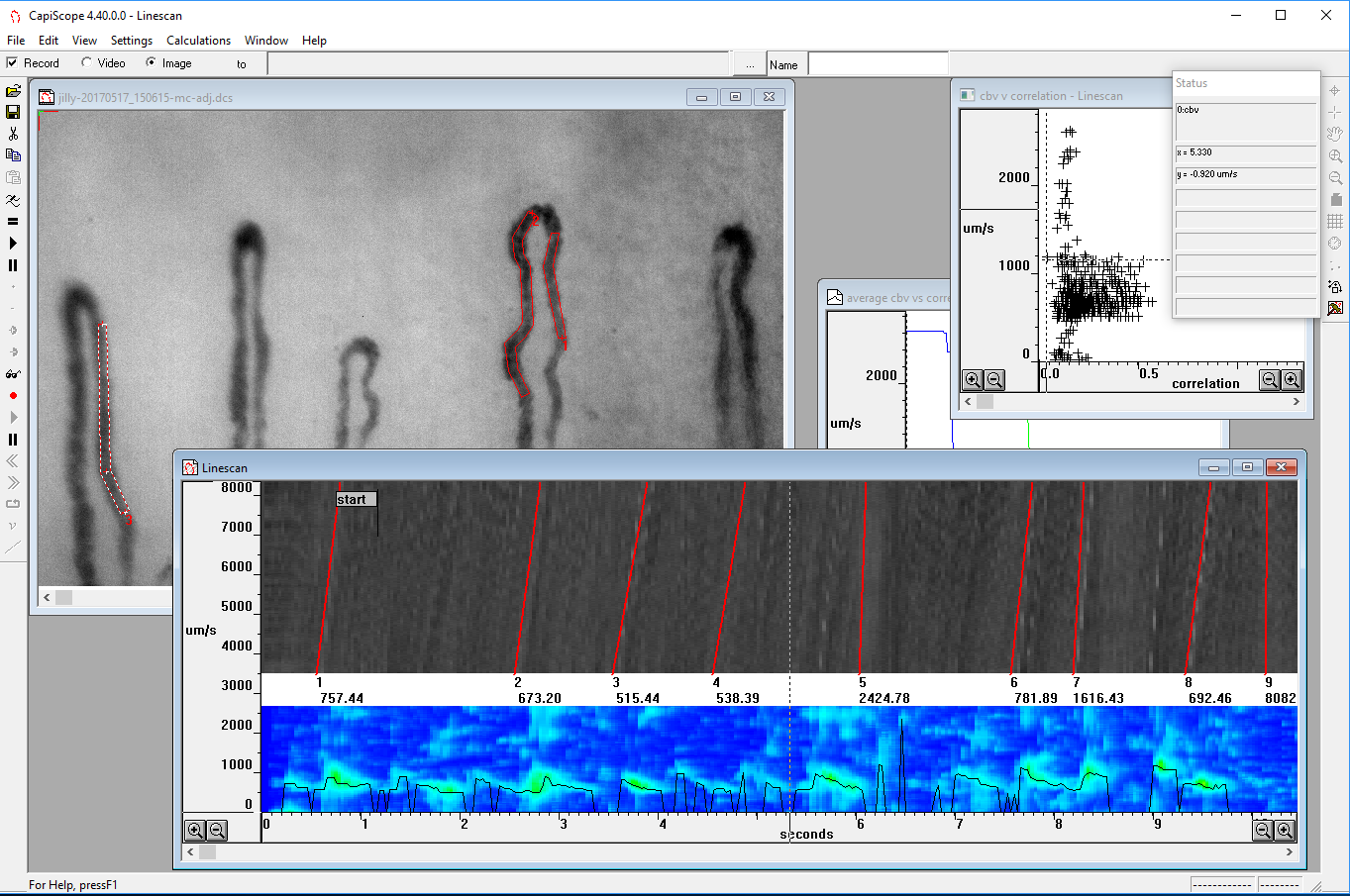
- Blood cell velocity from 0 to about 2mm/s using video correlation.
- Fast response time follows cardiac and vasomotion velocity changes.
- Interchangeable lenses available.
The CapiScope HVCS uses oblique profiled epi-illumination imaging (OPEI) to provide high contrast visualisation of the microcirculation in mucosal and other tissues.
Video and Image Recording
The CapiScope HVCS can display and record video at full frame and full frame rate. The monochrome video is stored uncompressed to avoid problems with information loss found with many digital video recorders. Single frames can be saved in bmp or tiff formats and video can be exported as avi files.
SPECIFICATIONS
Specifications
Imaging
| HVCS | HVCS-HR | HVCS-HR3 | |
| Image size (pixels) | 752 x 480 | 1280 x 1024 | 2048 x 1536 |
| standard lens: | |||
| Magnification (µm/pixel) | 0.92 | 0.81 | 0.546 |
| field of view (µm) | 692 x 442 | 1037 x 829 | 1118 x 839 |
| low power lens: | |||
| Magnification (µm/pixel) | 1.94 | 1.71 | 1.14 |
| field of view (µm) | 1459 x 931 | 2186 x 1749 | 2333 x 1750 |
Illumination: 4 x 525 nm light sources.
Video output: USB uncompressed.
Dimensions
Body: 100 mm x 42 mm Diameter
cap length: 129.5 mm
cap diameter at tip: 10mm
weight 270g
Operating Conditions
Temperature +10oC to +35oC
Relative Humidity 30% to 75% noncondensing
Altitude 700 to 1060 hPa
Shipping and Storage Conditions
Temperature -10:sup:oC to +45oC
Relative Humidity 95% noncondensing maximum
Software
CapiScope software requires Windows 10 or Windows 11.
Standards
Note
This is not a medical device, it has no diagnostic nor therapeutic function. For research use only (except in the UK excluding NI).
EN 60601-1:2006 /AC:2010 /A1:2013 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance
EN 60601-1-2:2007 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests
EN ISO 14971:2012 Medical devices - Application of risk management to medical devices.
EN 60825-1:2007 Safety of laser products. CLASS 1.
UKCA UK MDR 2002 (SI 2002 No. 618) [ Class 1 non-sterile ]
All devices prior to 2021-01-01: CE Medical Devices Directive 93/42/EEC Annex VII. [ class I device non-sterile ].
Intended Use
The CapiScope Video Microscope is intended solely for the visualization and characterization of the microcirculation system of the surface of mucosal and other tissues. No diagnostic or therapeutic claims are made for the device.
|Welcome| - |CapiScope CAM1 Capillary Anemometer| - |CapiScope HVCS Video Capillaroscopy| - |Sample Images and Videos| - |User Guides| - |References| - |Customers| - |Downloads| - |Contacts|
Copyright 2024 KK Technology
KK Research Technology Ltd, Honiton, Devon, EX14 1YL, England, United Kingdom, +44 140446242